Project Update
Earlier in the summer, we found that we can decompose urea into ammonia. However, we wanted to quantify the ammonia production in different ways. So last week, we set up one huge experiment with multiple parts.
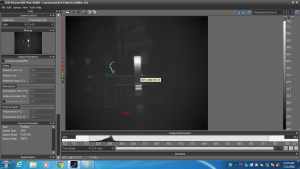
We repeated the use of an infrared laser to vaporize the urea, but we added a thermal camera and a balance. The thermal camera allowed us to measure the temperature of the solution in real-time. The balance allowed us to measure the amount of mass lost due to vaporization as we heat the urea solution.
The laser heated the solution, but only on the surface (the brighter portion of the image below); the rest of the sample remained close to room temperature!
Field Trip!
On Monday we took a trip to a water treatment plant. It was really interesting to see how many steps are involved in purifying water from the different lakes around Houston. One thing I did notice was the focus on removing the solid particles, then the treatment for pathogens. Nanotechnology would be a great bridge between the two steps, and may also help remove the pathogens altogether.
At the end of the tour, we did a scavenger hunt and I found these posters:
These would be a great way to have students present information they have learned! For example, my Physics students could make wanted posters of Physics laws or famous physicists.