This week has been an absolute whirlwind! We’re on the home stretch, friends.
So this week I’ve been splitting my time in the lab with working on my poster and getting feedback from the other people in the ball lab. Cody and Patrick have been especially helpful in the formation of the poster. Thanks guys!
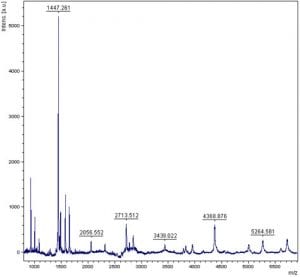
In the lab, we’ve been working on getting some protein for our reactions – with varying results. This is my mentor’s first time doing this, so the first result was a vial full of dead cells. Ooops! The next batch was quite a bit more successful and there was much fanfare, and we were able to culture the cells with some help from Chi’s lab.
Next we had to lyse the cells to get them to express the proteins, then centrifuge the crap out of them. There was quite a bit of back and forth as to how we wanted to split the cells up, but we ended up splitting them into 13 vials (which required a LOT of fiddling to get the mass within one gram of each other).

After lysing the cells and centrifuging them, we extracted the liquid and got some decent proteins! Katie also set up a boronic acid reaction without any oxygen present which I thought looked neat. There’s nitrogen in the balloon, which keeps the pressure above 1 atmosphere.
I got my first draft of my poster done though, and good lord that was a lot more work than I thought it was going to be! I’m going to be relieved when the poster is printed out and I get to see it in all its glory.