My first week as a NEWT-RET intern can be summed up as “Learn, Understand, Ask Questions, and Try It Out!” But that’s a little short for this blog post, so I should go back and explain.
This summer, I am working in Dr. Naomi Halas’ lab, with the goal to decompose urea into ammonia and carbon dioxide using aluminum nanoparticles and solar energy. Decomposition of urea into ammonia is already used in several applications, from plastic production to removing pollutants from diesel fuel. Ammonia is what is needed, but it’s difficult to store. Urea is simple to store and use, and you have the added benefit of using a waste material (urine) to make a compound that’s needed everywhere. Adding nanoparticles into the system will allow for this decomposition to occur without spending extra money on the energy required. The sun will provide all the energy we need.
To accomplish this goal, I am working with my mentor, Dr. Oara Neumann. I absolutely love her teaching style! It is not enough to just be able to read the procedure and run the experiments. She wants me (and everyone else in the lab) to understand the underlying principles. Do we understand why we’re doing these experiments? What is the theory behind it? How do we get from theory to practice?
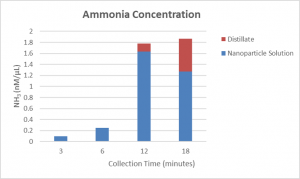
So the first step for me was to understand how urea decomposes into ammonia. Then I spent time in the lab, learning how to create different types of nanoparticles, aluminum-MOFs (aluminum particles coated with metal-organic frameworks) and nano matroyshkas (layered nanoparticles, named after the stackable dolls). I observed the construction of the solar module we would be using. We even created a calibration curve so we could determine the concentration of ammonia we obtain!
Throughout each step, I would have questions. Dr. Neumann teaches me the same way I teach my students: answer a question with a question. It’s easy to just give someone the answer, but then they won’t really learn or understand. It’s better to help them work toward the answer. Give them questions that would help them find the answers they seek. She has helped me gain a much better understanding of the process, especially the chemistry!
The real trick is going to be the use of this little guy:
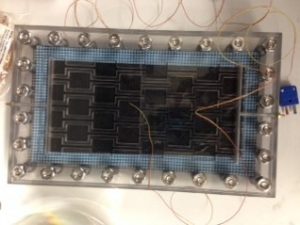
This is the solar module that will hold a membrane coated with the aluminum-MOF nanoparticles. As the sun shines on it, the nanoparticles will absorb and resonate that energy to the surrounding solution, causing it to turn to vapor before the entire solution is even able to boil. The ammonia vapor will pass through the membrane and condense, so we can collect it. It’s great! It’s fantastic! It requires a sunny day, and it’s going to rain for a full week….
Not a problem. We will just have everything ready and run out into the sun.