Since here at NEWT we are all working on water projects. I have started to wonder about the water we drink and I asked my mentor what type of water he drinks and if he has ever tested his water at home to see what’s in it. He said at his place he drinks from the tap. He said he has a filter, but it takes too long. I find it interesting that most of us don’t worry about our water supply, but I know a lot of people who won’t drink tap water. There is a hesitation about what’s really in the water and it is perceived as not truly being pure. I believe situation like lead found in Flint, Michigan and even chromium-6 found here in Houston’s water supply in Alief doesn’t help.
This week we were able to go on a field trip to get a glimpse of how the City of Houston provides it residence with drinking water.

We learned there are 3 purification plants in the city and we were at the largest. They are able to produce 80 million gallons a day and after an expansion they will attempt to produce 400 million gallons a day!


We were able to complete a scavenger hunt and an activity covering the water cycle. We were also given free samples!

I was also able to visit the Buffalo Bayou Cistern.

“The Buffalo Bayou Park Cistern is a former drinking water reservoir built in 1926 for the City of Houston. As one of the city’s early underground reservoirs, it supported the municipal water system’s goals of fire suppression (water pressure) and drinking water storage. After operating for decades, an irreparable leak was discovered and after a few years, the reservoir was decommissioned in 2007.” So if you lived or visited Houston prior to 2007, you probably had water that was stored in this facility. Information about Buffalo Bayou
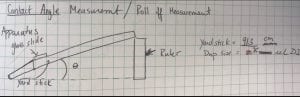
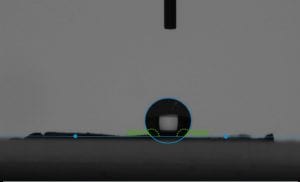
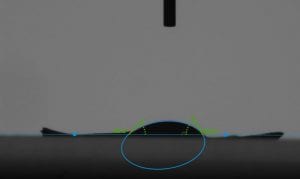
This week I was also able to run tests on my final membrane. We continued to test the contact angle and so far we have hydrophobic membrane, but it is not quite superhydrophobic. Next week, we plan to complete our journey to superhydrophobicity by adding silver nanoparticles and coating it with chemical to lower surface energy.
Make sure you all answer my question about what is in your water or what type of water you drink in comment section.
Currently, I use the tap for cooking and all of my other water needs, but I drink bottled or filtered tap water. Where does your water come from? What kind do you drink? Tap, Filtered Tap, Bottled, Purified, Spring?